Solved 1.What Are The Three Types Of Bonding? 2.What Does...
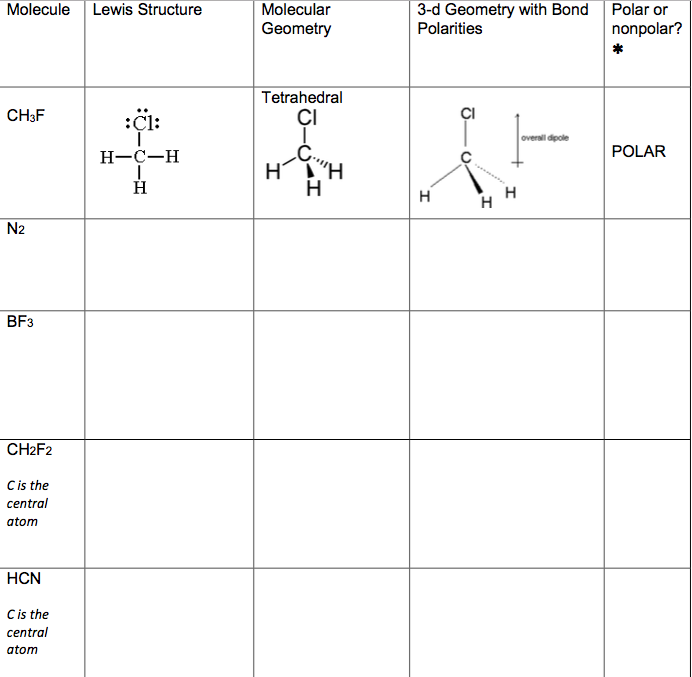
An explanation of the molecular geometry for the CH2F2 (Difluromethane) including a description of the CH2F2 bond angles. The electron geometry for the Difluromethane is also provided..
[Solved] Please slove. Question 52 3 pts The molecular geometry of the
Learn to determine if CH2F2 (Dichloromethane) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis.
Ch2f2 Lewis Structure
The CH2F2 molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the CH2F2 molecule in a specific geometric manner.
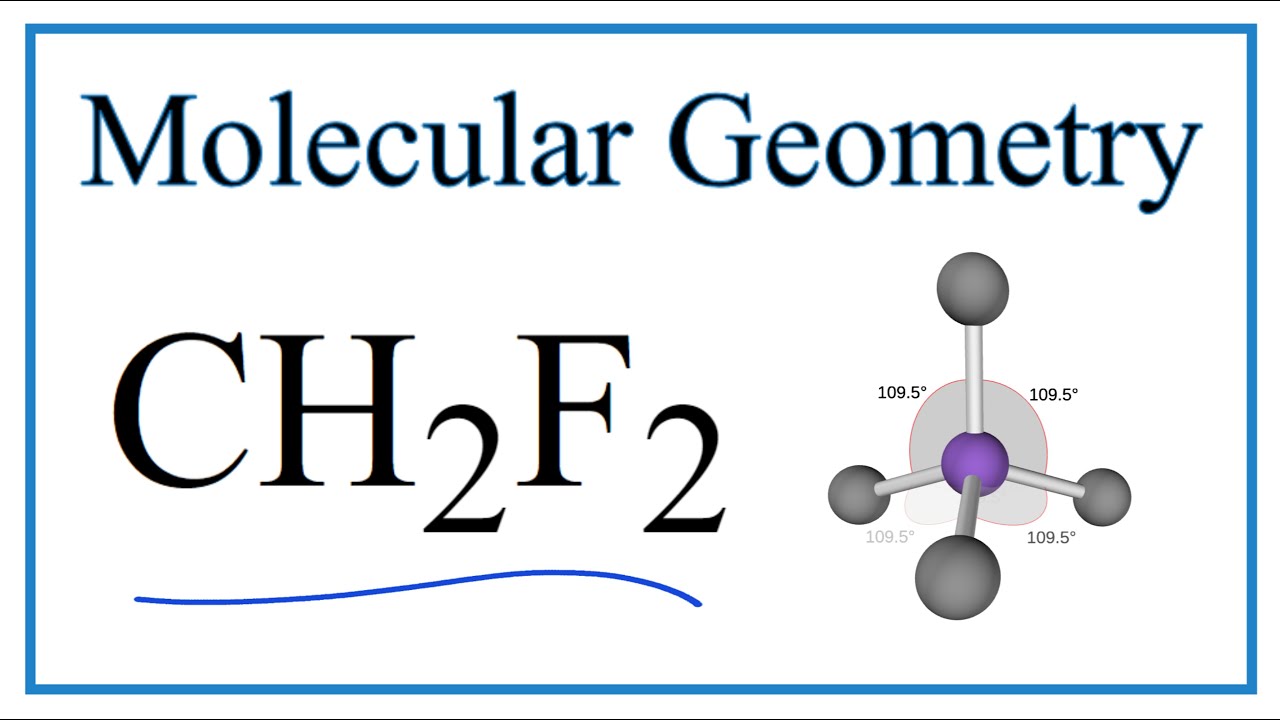
CH2F2 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
Difluoromethane, also called difluoromethylene, HFC-32 Methylene Fluoride or R-32, is an organic compound of the dihalogenoalkane variety. It has the formula of CH 2 F 2. It is a colorless gas in the ambient atmosphere and is slightly soluble in the water, with a high thermal stability.
Ch2f2 Shape
∴ Total number of valence electrons available for the CH 2 F 2 Lewis structure = 4 + 1 (2) + 7 (2) = 20 valence electrons [∴ CH 2 F 2 molecule has one carbon, two hydrogen, and two fluorine atoms] 2. Find the least electronegative atom and place it at center

CH2Br2 Lewis Structure How to Draw the Lewis Structure for CH2Br2
This is the highest occupied molecular orbital at orbital 13. The orbitals were calculated by summing the amount of electrons in the molecule and dividing by two. Electrostatic potential This is the electrostatic potential of the molecule.

[Solved] CH2F2 Draw the lewis dot structure Dete
CH2F2 is industrially manufactured via the fluorination of Dichloromethane (CH2Cl2). Dichloromethane reacts with Hydrogen Fluoride (HF) in three phases under different conditions. Owing to its thermodynamic properties, Difluoromethane is used as a refrigerant. CFCs and other aerosols have been ruled as damaging to the ozone layer.

Ch3cooh Molecular Geometry
Figure 5.2.9 5.2. 9: (a) H2O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. (b) Two of the electron regions are lone pairs, so the molecular structure is bent. Exercise 5.2.3 5.2. 3. The hydronium ion, H 3 O +, forms when acids are dissolved in water.

CH2Cl2 Molecular Geometry Science Education and Tutorials
1. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is. Three fluorines are bonded to a central bromine. Each fluorine has three lone pairs, Bromine has two lone pairs. Once again, we have a compound that is an exception to the octet rule. 2.
[Solved] Please slove. Question 52 3 pts The molecular geometry of the
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The DIFLUOROMETHANE molecule contains a total of 4 bond (s). There are 2 non-H bond (s). Images of the chemical structure of DIFLUOROMETHANE are given below: 2-dimensional (2D) chemical structure image of DIFLUOROMETHANE.

Ch2F2 Molecular Geometry
A step-by-step explanation of how to draw the CH2F2 Lewis Dot Structure (Difluoromethane).For the CH2F2 structure use the periodic table to find the total nu.

Sf2 molecular geometry zeseoseouc
Hello Guys!CH2F2 or Dichloromethane has a quite simple structure as it consists of one central carbon atom forming single covalent bonds with two hydrogen an.
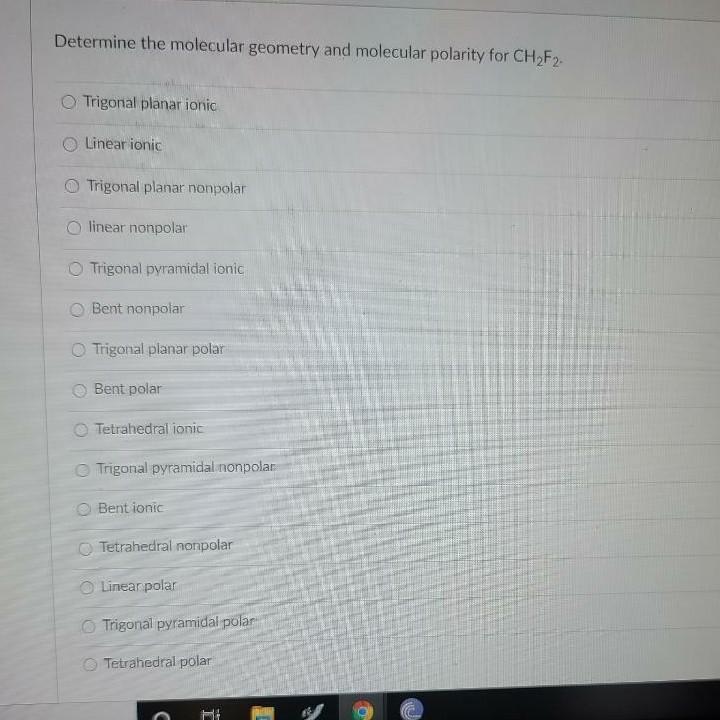
Solved Determine the molecular geometry and molecular
Molecular Model. Structural Formula. CH 2 F 2. difluoromethane . Molecular Model Application loaded..
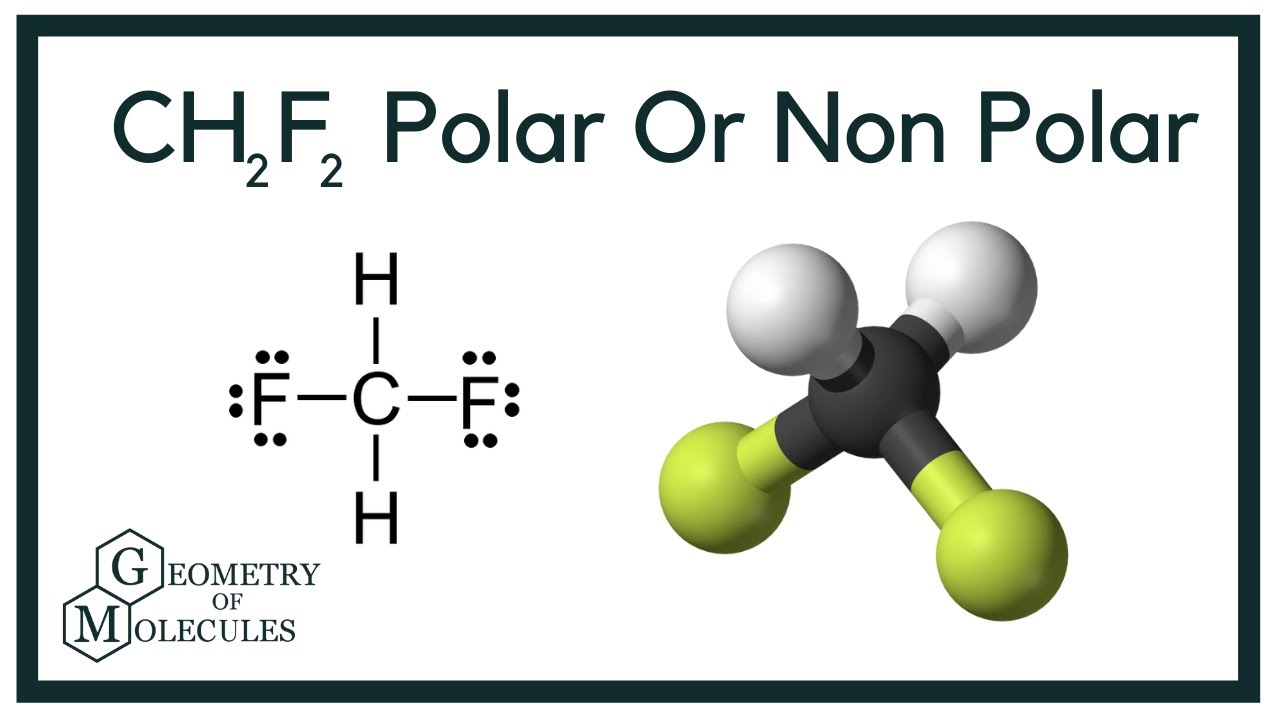
Is CH2F2 Polar or Nonpolar? (Difluoromethane) YouTube
In the CH 2 F 2 Lewis structure, there are four single bonds around the carbon atom, with two hydrogen atoms and two fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs. CH2F2 Lewis Structure - How to Draw the Lewis Structure for CH2F2 Watch on Contents Steps #1 Draw a rough skeleton structure

Top Ch2F2 Molecular Geometry The Latest GM
Let's do the Lewis structure for CH2F2: difluoromethane. On the periodic table, Carbon is in group 4, it has 4 valence electrons. Hydrogen, group 1, but we have 2 Hydrogens. Fluorine, 7 valence electrons, we have 2 of those as well, for a total of 20 valence electrons. We'll put the Carbon at the center, and then Hydrogens always go on the outside.
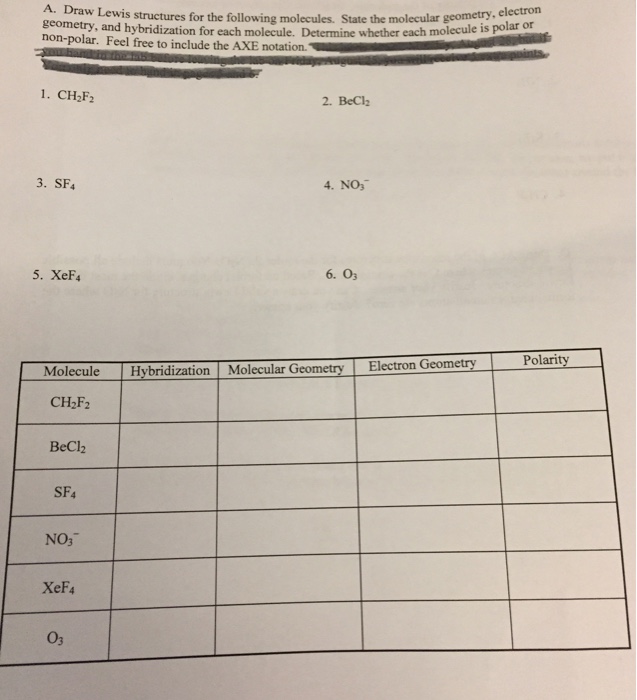
Solved Draw Lewis Structures For The Following Molecules....
CH2F2 is a POLAR molecule because the C-F bonds present in the molecule are polar, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire CH2F2 molecule polar.