
Solved 1. Aspirin can be made in a laboratory by reacting
acetic anhydride - cas 108-24-7, synthesis, structure, density, melting point, boiling point
[Solved] The density of acetic anhydride is 1.08g/ml calculate the mass of... Course Hero
Acetic anhydride Formula: C 4 H 6 O 3 Molecular weight: 102.0886 IUPAC Standard InChI: InChI=1S/C4H6O3/c1-3 (5)7-4 (2)6/h1-2H3 IUPAC Standard InChIKey: WFDIJRYMOXRFFG-UHFFFAOYSA-N CAS Registry Number: 108-24-7 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file
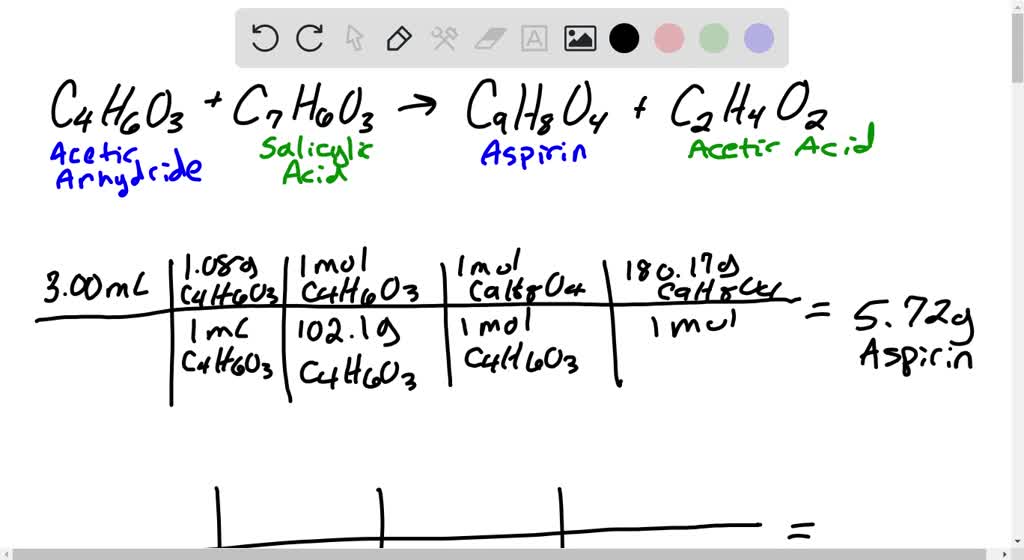
SOLVED Aspirin can be synthesized by the following balanced reaction CHICOCoH COH CH;COOH (CH
Acetic anhydride is an organic compound that is composed of carbons, hydrogens, and oxygens. This organic molecule is the simplest carboxylic acid anhydride, which is a functional group that.

Solved CEIIIL PrelabAspirin Name 1. Acetic anhydride is a
Acetic anhydride Write a review 99.5% Synonym (s): Acetanhydride, Acetic acid anhydride, Acetyl acetate, Acetyl anhydride, Ethanoic anhydride Linear Formula: (CH3CO)2O CAS Number: 108-24-7 Molecular Weight: 102.09 Beilstein: 385737 EC Number: 203-564-8 MDL number: MFCD00008705 PubChem Substance ID: 24878429 NACRES: NA.21

acetic anhydride vapor density
The molar mass of acetic anhydride is 102.1 g/mol and its density is 1.080 g/mL? Solution The formula for density is ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a ρ = m V a a ∣∣ −−−−−−−−−−−− where ρ is the density, m is the mass, and V is the volume of the sample. We can rearrange the formula to get m = V ×ρ mass = 10.00mL × 1.080 g 1mL = 10.80 g

Solved (a) How many moles of acetic anhydride were replaced
Acetic anhydride Formula: C 4 H 6 O 3 Molecular weight: 102.0886 IUPAC Standard InChI: InChI=1S/C4H6O3/c1-3 (5)7-4 (2)6/h1-2H3 IUPAC Standard InChIKey: WFDIJRYMOXRFFG-UHFFFAOYSA-N CAS Registry Number: 108-24-7 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file

Acetyl Chloride Uses Chemistry notes, Acetic anhydride, Chemistry
Description Acetic anhydride appears as a clear colorless liquid with a strong odor of vinegar. Flash point 129 °F. Corrosive to metals and tissue. Density 9.0 lb /gal. Used to make fibers, plastics, pharmaceuticals, dyes, and explosives. CAMEO Chemicals Acetic anhydride is an acyclic carboxylic anhydride derived from acetic acid.
[Solved] Appearance of acetic anhydride Volume of acetic anhydride Density... Course Hero
Acetic anhydride Formula: C 4 H 6 O 3 Molecular weight: 102.0886 IUPAC Standard InChI: InChI=1S/C4H6O3/c1-3 (5)7-4 (2)6/h1-2H3 IUPAC Standard InChIKey: WFDIJRYMOXRFFG-UHFFFAOYSA-N CAS Registry Number: 108-24-7 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file

Acetic Anhydride 500ml
Ethanoic anhydride is a colorless liquid, smelling strongly of vinegar (ethanoic acid). The smell is because ethanoic anhydride reacts with water vapor in the air (and moisture in your nose) to produce ethanoic acid again. This reaction with water is given in detail on another page. (Find it from the acid anhydrides menu - link at the bottom of.

Acetic Anhydride Structure Acetic anhydride, Math equations, Chemistry
IUPAC identifier Office of Data and Informatics IUPAC Standard InChI:InChI=1S/C4H6O3/c1-3 (5)7-4 (2)6/h1-2H3 IUPAC Standard InChIKey:WFDIJRYMOXRFFG-UHFFFAOYSA-N CAS Registry Number: Chemical structure: This structure is also available as a 2d Mol file 3d SD file The 3d structure may be viewed using

1. What precautions should you take when working
Density (1) 1.082 g/cm³ @ Temp: 20 °C; Source(s). Acetic anhydride; Acetic acid, anhydride; Acetic oxide; Acetyl oxide; View All. CAS INSIGHTS TM. CAS Common Chemistry is provided under the Creative Commons CC BY-NC 4.0 license. By using CAS Common Chemistry, you agree to the terms and conditions of this license.
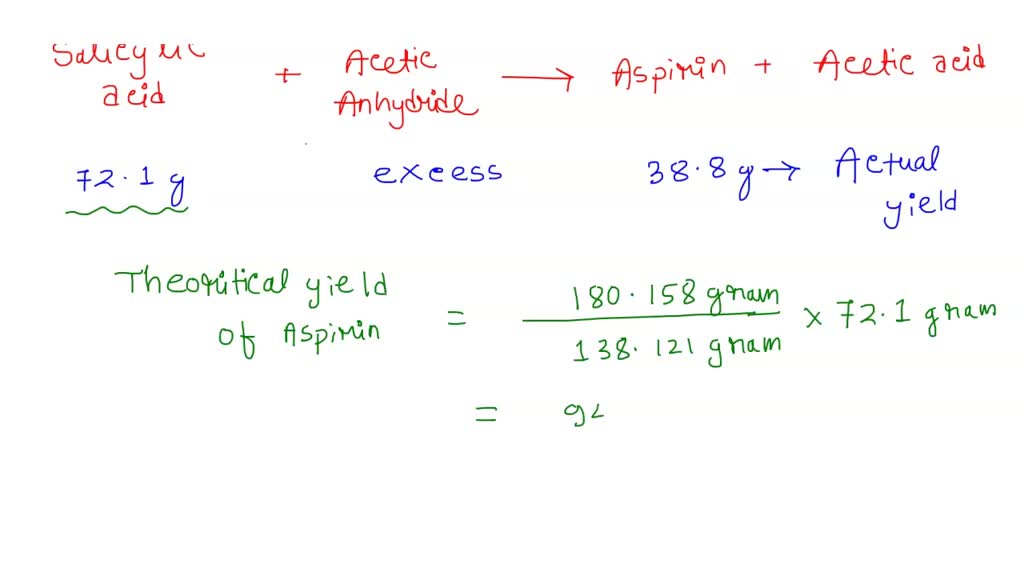
SOLVED Aspiring (CoHgO4) is made by reacting salicylic acid (C,HsO3) with acetic anhydride
C p,liquid: Constant pressure heat capacity of liquid: P c: Critical pressure: T: Temperature: T boil: Boiling point: T c: Critical temperature: T fus: Fusion (melting) point: Δ f H° liquid: Enthalpy of formation of liquid at standard conditions

acetic anhydride chemical formula
Description Acetic anhydride (chemical formula: (CH3CO)2O) is the simplest isolable anhydride of a carboxylic acid, is widely used as a reagent in organic synthesis. It has an internal asymmetric structure, leading to its potent electrophilicity.

Preparation of Acetic Anhydride Acetic anhydride, Chemistry notes, Sodium acetate
Being a carboxylic acid anhydride, acetic anhydride will slowly decay in contact with water into acetic acid. This process, however, is slow enough for aqueous solutions to be made and used immediately. Physical. Acetic anhydride is a colorless liquid with a density slightly greater than that of water.

Acetic Anhydride, Reagent Grade, 100 mL
Vapor Density: 3.5 (air=1) Evaporation Rate:0.46 (n-butyl acetate=1) Viscosity: 0.91mPa.s @ 20 deg C Boiling Point: 140 deg C @ 760mmHg. Since acetic anhydride is a relatively non-volatile liquid, direct venting of the vapor to the atmosphere from a hole in a ruptured vessel does not consitiute a significant hazard downwind. Only vapor.

Acetic Anhydride Density, Formula & Uses Video & Lesson Transcript
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac 2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis.