
Fcn Lewis Dot Structure Abbreviations Fch Family Cohesion Fcn Family
Steps of drawing FCN lewis structure Step 1: Find the total valence electrons in FCN molecule. In order to find the total valence electrons in an FCN molecule, first of all you should know the valence electrons present in fluorine atom, carbon atom as well as nitrogen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

How to Draw the Lewis Dot Structure for FCN Cyanogen Fluoride YouTube
FCN is a chemical formula for cyanogen fluoride. And to help you understand the Lewis Structure of this molecule, we are going to share our step-by-step met.
lewis dot structure chart Focus
FCN lewis structure, also known by the chemical name as cyanogen fluoride, is an inorganic molecule with a molecular weight of 45.016 g/mol. Some facts about FCN lewis structure : Molar mass/Molecular weight = 45.016 g/mol. Boiling point = -46.17 0 C , Melting point = - 82 0 C .
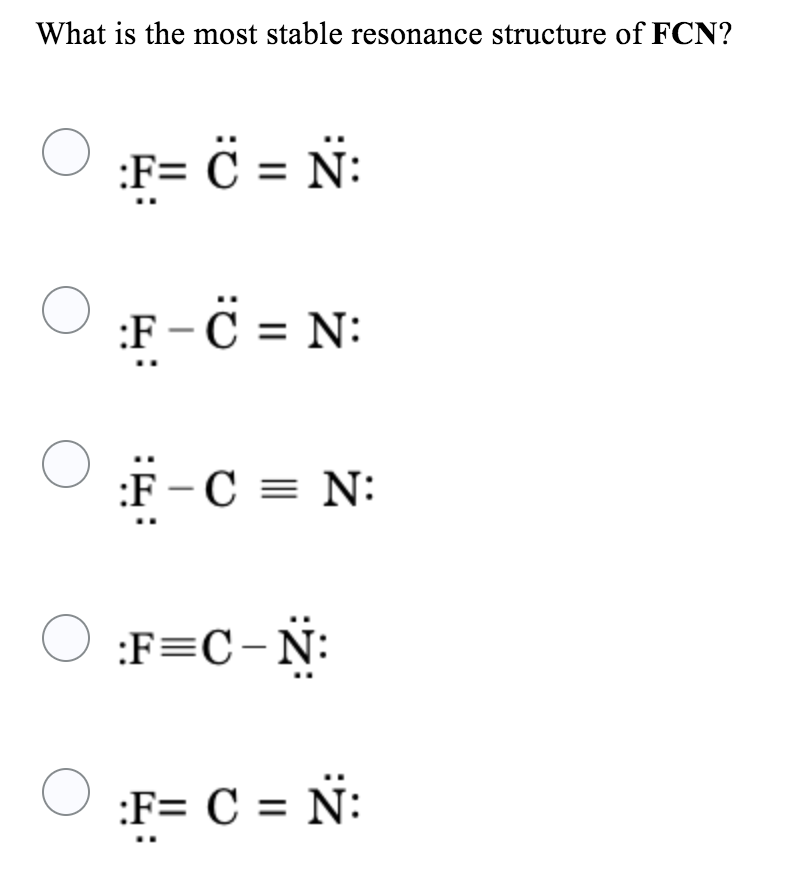
Solved What is the most stable resonance structure of FCN?
A step-by-step explanation of how to draw the CN- Lewis Dot Structure (Cyanide ion).Note: there should be brackets around the final Lewis Structure with a -1.

[DIAGRAM] Kcl Lewis Dot Diagram
A step-by-step explanation of how to draw the FCN Lewis Dot Structure.For the FCN structure use the periodic table to find the total number of valence electr.

draw the main lewis structure of nofnof. darnelllemmings
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Draw Lewis Structure
Choose the best Lewis structure for FCN and explain why it is the best C==N: 6_C=n: Structure is better because it is symmetrical with 2 double bonds Structure b is better because it has all formal charges that are zero while structure has a + | charge on the N and a - ] charge on the F
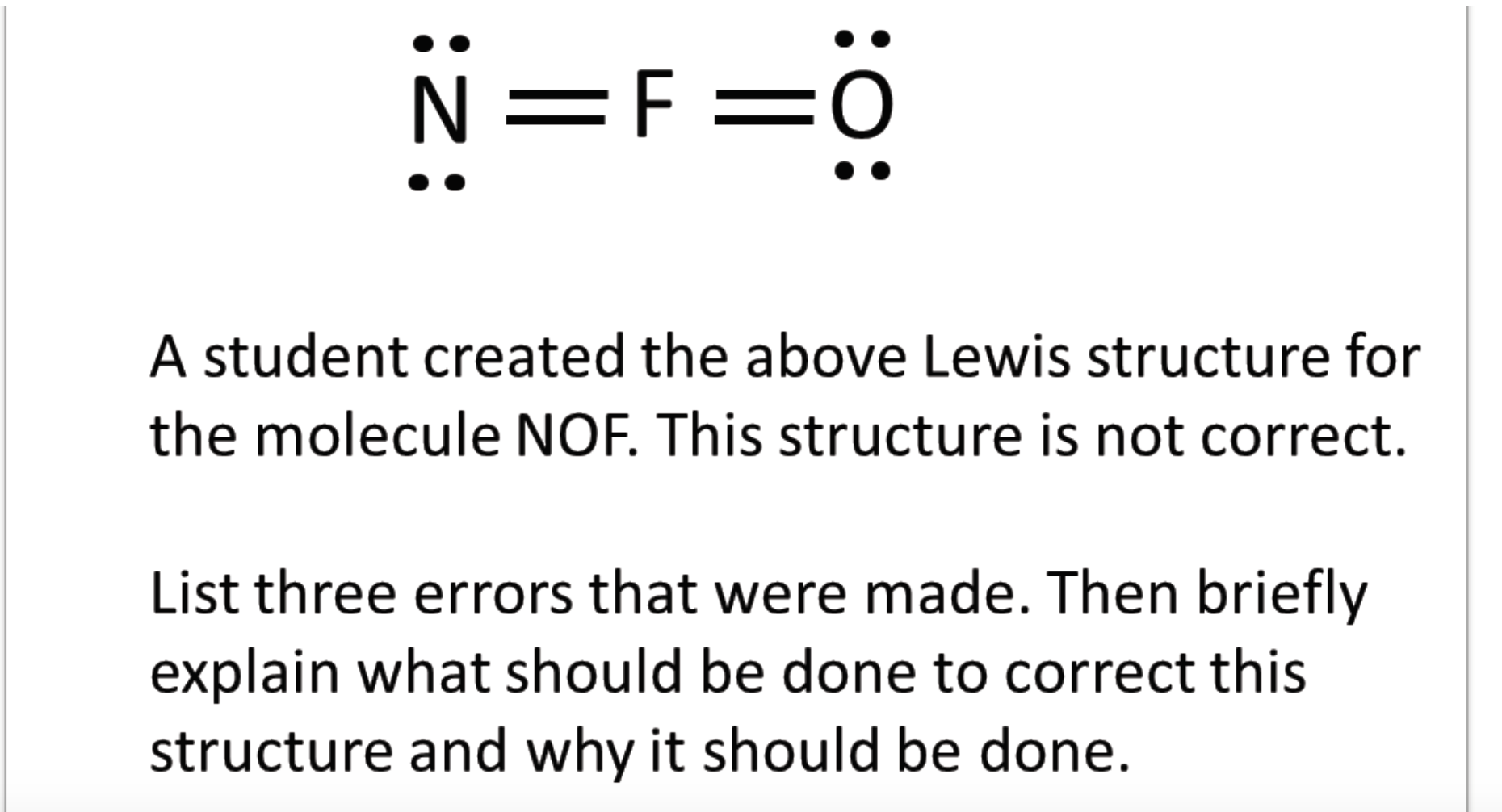
Solved N=F =0 A student created the above Lewis structure
Summary. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. There are three exceptions: (1) When there are an odd number of valence electrons, (2) When there are too few.
:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)
Lewis Structure Definition and Example
What is the Lewis structure of [//substance:FCN//]? Natural Language; Math Input; Extended Keyboard Examples Upload Random. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports.
[Solved] 1. Draw Lewis structures for each of the following. Use VSEPR
In FCN Lewis structure, there is a single bond between carbon and fluorine atom, and a triple bond between carbon and nitrogen atom. The fluorine atom has three lone pairs, and the nitrogen atom has one lone pair. Contents. Steps #1 First draw a rough sketch
Draw Lewis Structure
Chemistry questions and answers. Draw the correct three dimensional Lewis structure of FCN (Central atom is carbon) Must show all lone pairs with dots and bonded atoms with lines, Then answer the following questions: A. Electron pair geometry: B. Molecular geometry: C. Bond angle: D. Show polarity of bonds by using (delta notations) for partial.

FCN Lewis Structure How to Draw the Lewis Dot Structure for FCN
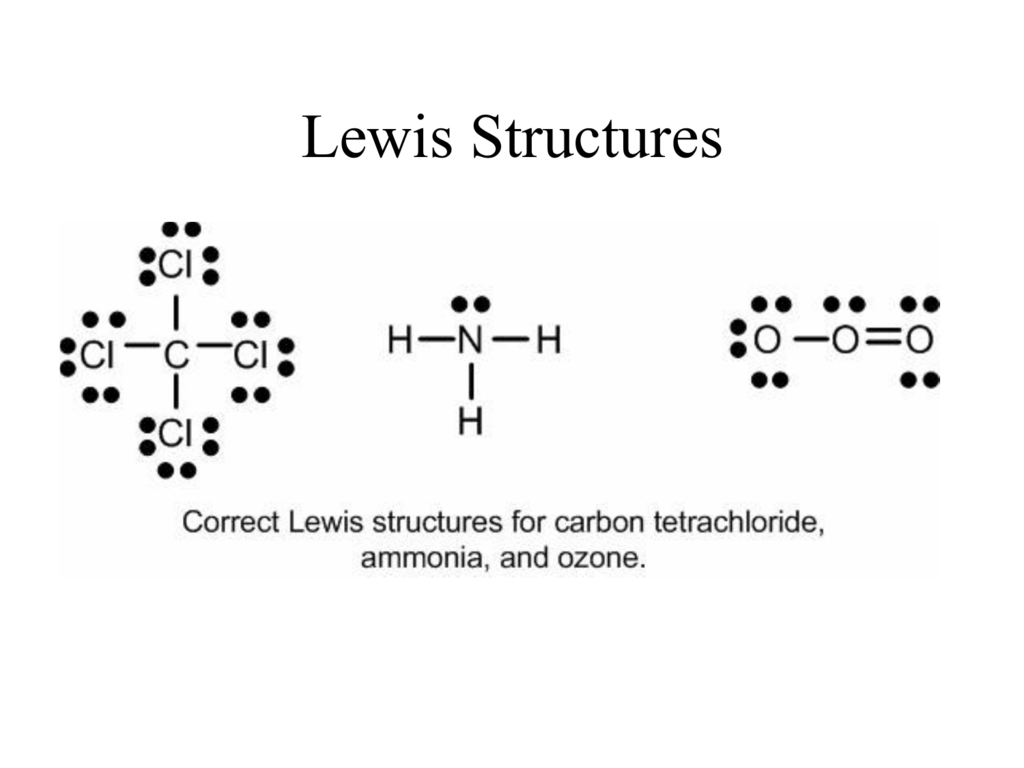
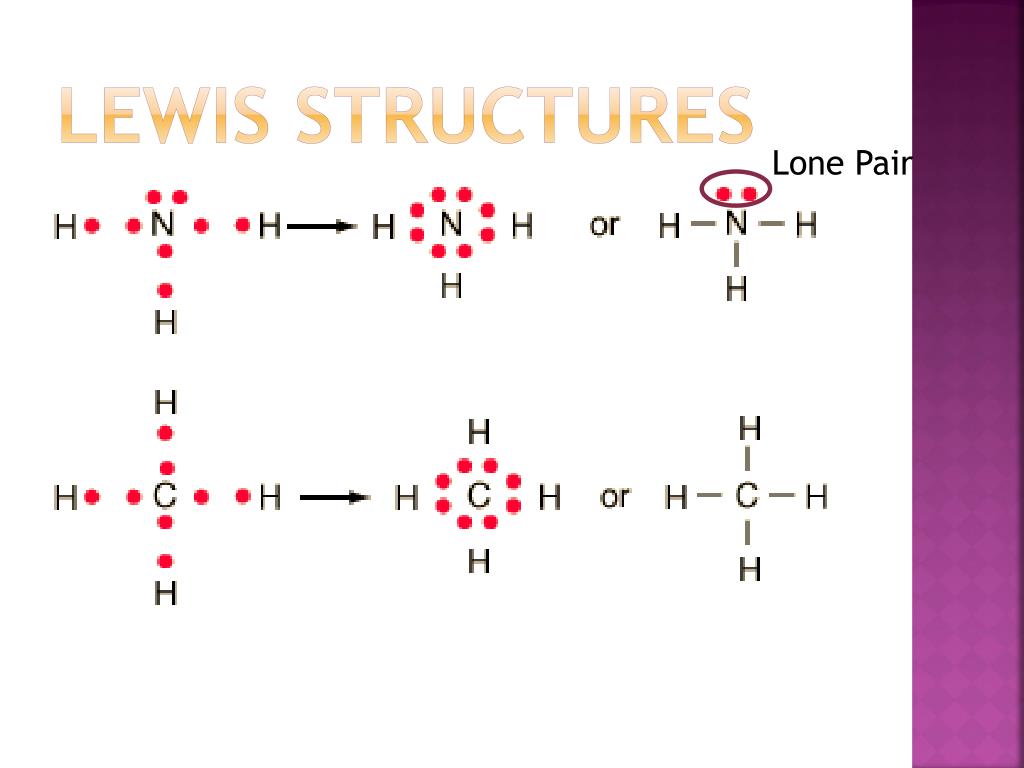
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
How To Draw 3D Lewis Structures Draw Lewis Structure C3h6 / Lewis
FCN Lewis Structure FCN is a chemical formula for cyanogen flouride. And to help you understand the Lewis Structure of this molecule, we are going to.

How To Draw Lewis Structures In Chemdraw Images and Photos finder
The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well.
Breathtaking Tips About How To Draw A Lewis Dot Structure Ballchicken
Strong electron delocalization in your best Lewis structure will also show up as donor-acceptor interactions. Interactions greater than 20 kJ/mol for bonding and lone pair orbitals are listed below. The interaction of lone pair donor orbital, 8, for F1 with the antibonding acceptor orbital, 70, for N2-C3 is 70.3 kJ/mol.
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
Best How To Draw The Lewis Dot Structure in the year 2023 The ultimate
Cyanogen fluoride (molecular formula: FCN; IUPAC name: carbononitridic fluoride) is an inorganic linear compound which consists of a fluorine in a single bond with carbon, and a nitrogen in a triple bond with carbon. It is a toxic and explosive gas at room temperature. It is used in organic synthesis and can be produced by pyrolysis of cyanuric fluoride or by fluorination of cyanogen.