10+ Lewis Dot Structure For Hcn Robhosking Diagram
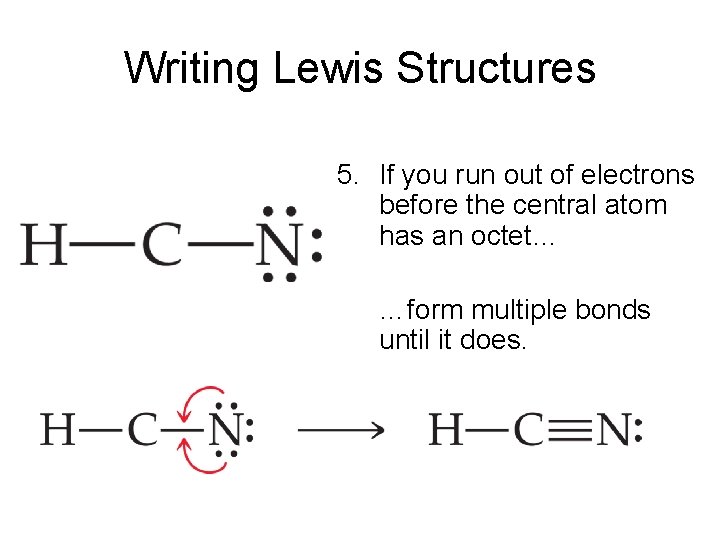
HCN Lewis dot structure consist of 3 elements as shown in the formula. Due to electronegativity difference carbon is the central atom which shares its 1 electron with hydrogen and 3 electrons with nitrogen to fulfill the stability criteria. This leads to formation of carbon forming single covalent bond with hydrogen and triple covalent bond.
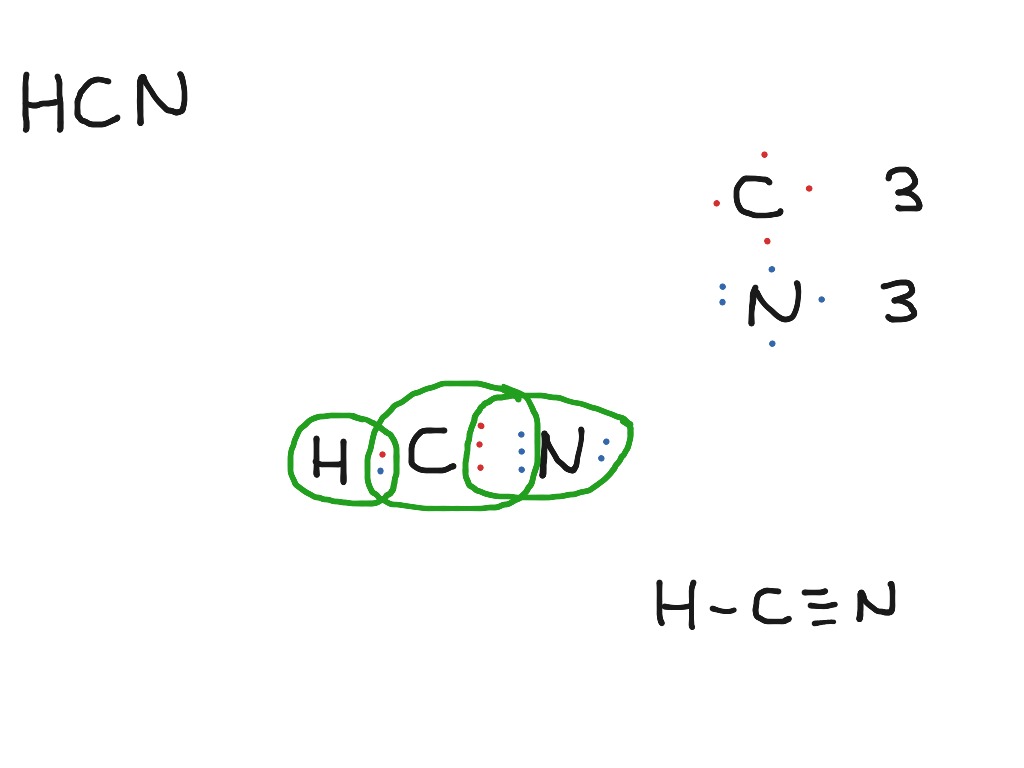
Electron dot diagram HCN Science, Chemistry, Molecules ShowMe
A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share.

HCN Lewis Structure HCNLewisStructure Lewis Dot Structure for HCN YouTube
Each hydrogen atom in the molecule shares one pair of bonding electrons and is therefore assigned one electron [0 nonbonding e − + (2 bonding e − /2)]. Using Equation 8.2.1 to calculate the formal charge on hydrogen, we obtain. formalcharge(H) = 1 valence e − − (0non − bonding e − + 2 bondinge − 2) = 0.
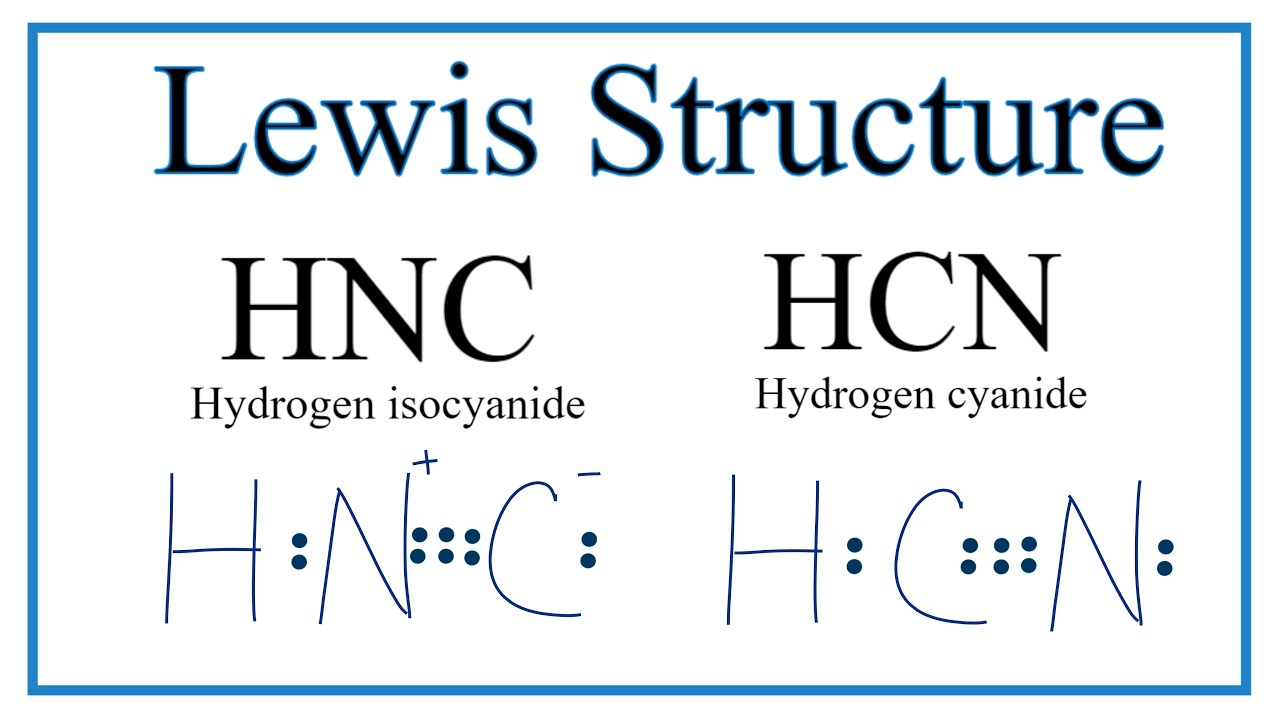
Hcn Lewis Structure Bonds Draw Easy
The Lewis dot diagram for HCN is as follows: The hydrogen atom is represented by a single dot, the carbon atom by four dots (arranged horizontally or vertically), and the nitrogen atom by five dots (arranged in a cross shape). These dots represent the valence electrons of each atom. The hydrogen atom shares its electron with carbon, and carbon.

HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw Lewis Structure of HCN
White phosphorus reacts spontaneously with the oxygen in air to form P4O6. (b) When P4O6 is dissolved in water, it produces a H3PO3 molecule. H3PO3 has two forms, P forms 3 covalent bonds in the first form and P forms 5 covalent bonds in the second form. Draw two possible Lewis structures of H3PO3. 394.

HCN Lewis Structure (Hydrogen Cyanide) Molecules, Chemical formula, Lewis
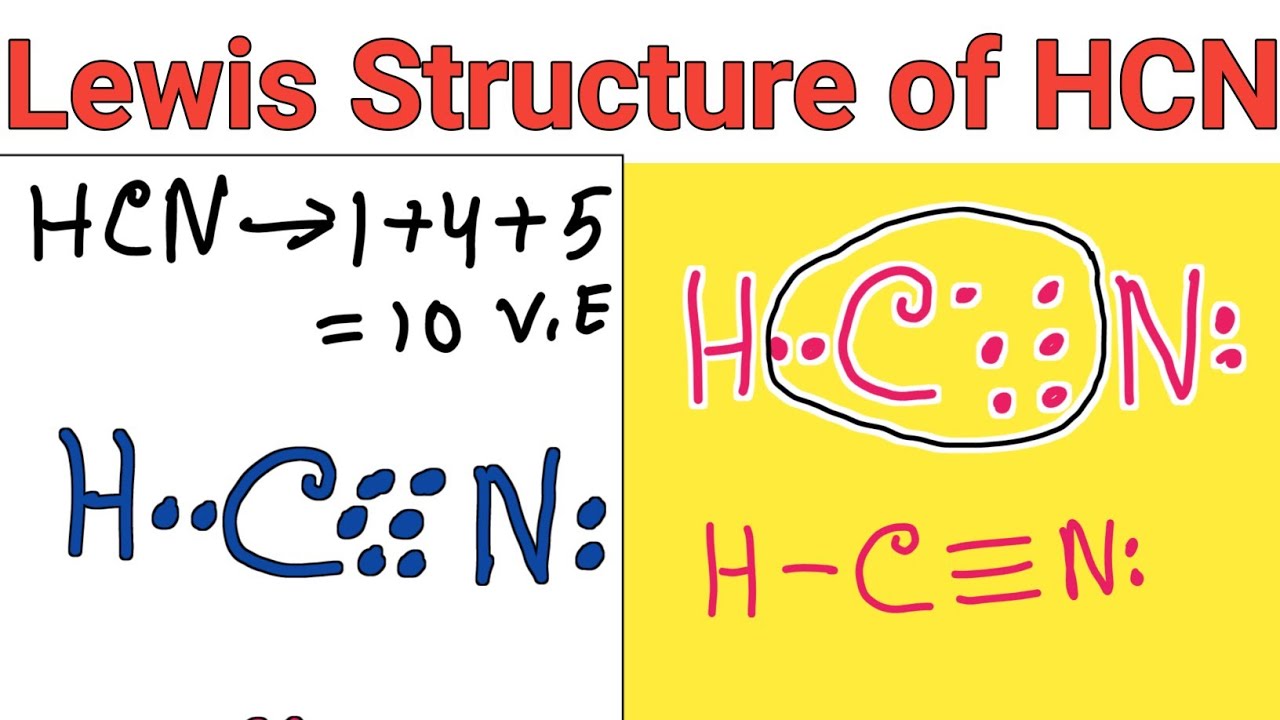
For the HCN Lewis structure, calculate the total number of valence electrons for the HCN molecule.
Lewis structure of HCN (Hydrogen cyanide) YouTube
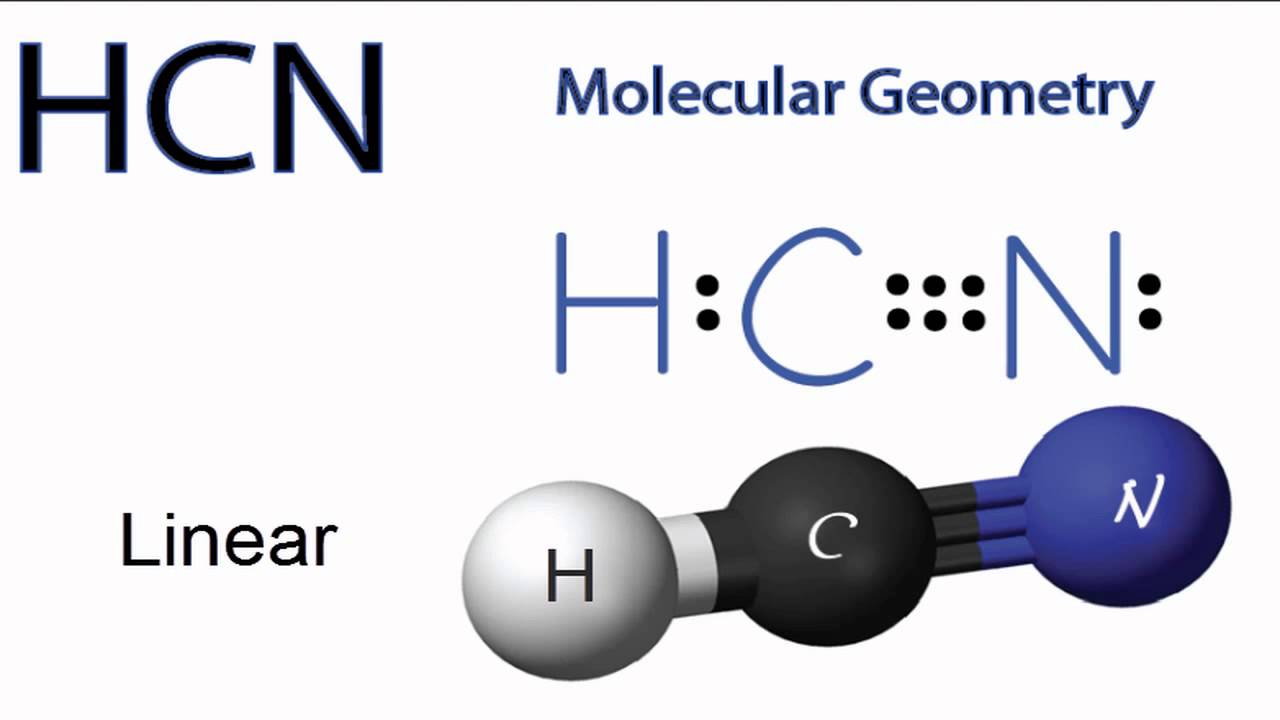
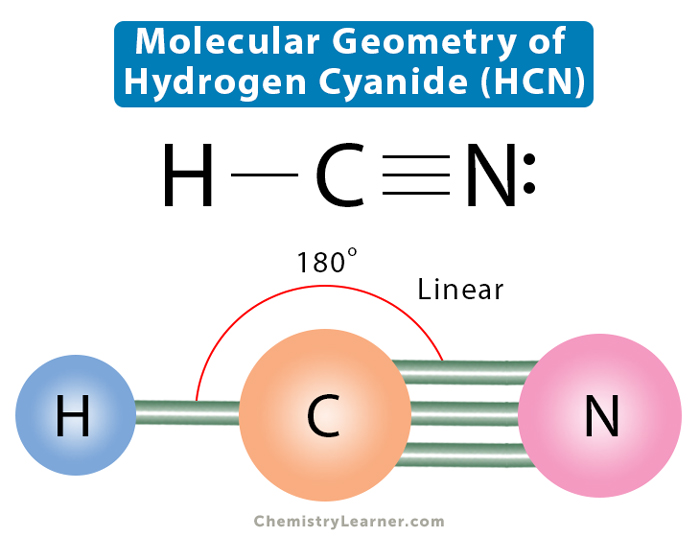
HCN is a highly toxic substance that has a bitter almond-like smell. There is one bond between H and C and three bonds between C and nitrogen. There is one lone pair of. electrons on the nitrogen atom. The compound has sp hybridization. The molecular geometry of HCN is linear. The compound is polar in nature.
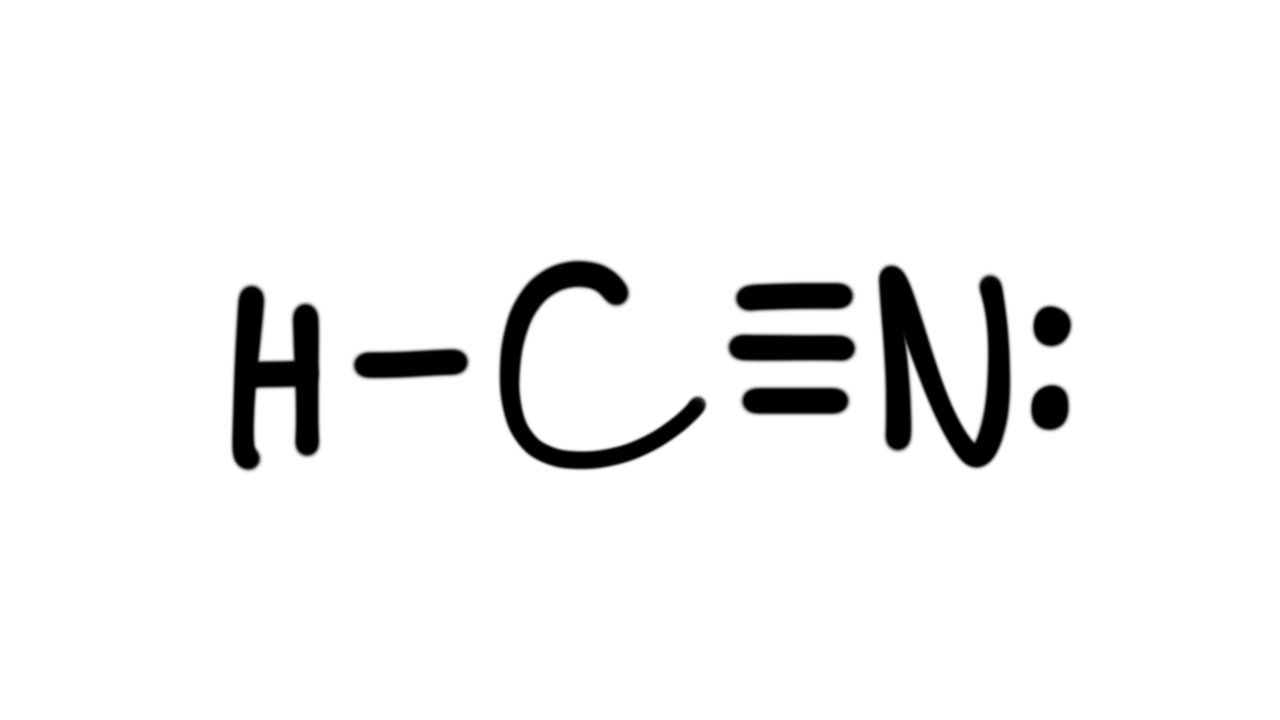
Lewis Diagram For Hcn
Draw the Lewis dot structure of Hydrogen cyanide (HCN) molecule .
[Solved] Draw the Lewis structure for it as well. 3. Hydrogen cyanide (HCN)... Course Hero
HCN, hydrogen cyanide, is a volatile and poisnous compound with distinguished bitter odor. It is linear molecule with a triple bond between C and N atom and has bond angle of 180 degrees.. The Valence Bond thoery simply explains the bond formation just like lewis dot structure, but instead it explains the bonding in terms of covalent bond by.
Hcn Lewis Structure Bonds Draw Easy
Step-1: HCN Lewis dot Structure by counting valence electrons on the carbon and nitrogen atom. To calculate the valence electron of each atom in HCN, look for its periodic group from the periodic table. The carbon, nitrogen, and hydrogen group families, which are the 17th and 1st groups in the periodic table, are both made up of carbon.
Lewis Dot Diagram For Hcn Wiring Site Resource
Steps. Use these steps to correctly draw the HCN Lewis structure: #1 First draw a rough sketch #2 Mark lone pairs on the atoms #3 Calculate and mark formal charges on the atoms, if required #4 Convert lone pairs of the atoms, and minimize formal charges #5 Repeat step 4 if needed, until all charges are minimized, to get a stable Lewis structure

So far, we’ve used 8 of the HCN Lewis structure’s total 8 outermost valence shell electrons. One
This video shows you how to draw Lewis Dot structure in 5 easy steps.1. Count total valence electrons for the molecule.2. Put the least electronegative atom.

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity Techiescientist
Step #1: Calculate the total number of valence electrons. Here, the given molecule is HCN. In order to draw the lewis structure of HCN, first of all you have to find the total number of valence electrons present in the HCN molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
Molecular Geometry, Lewis Structure, and Bond Angle of HCN
The Lewis Structure (Lewis Dot Diagram) for HCN.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill out.
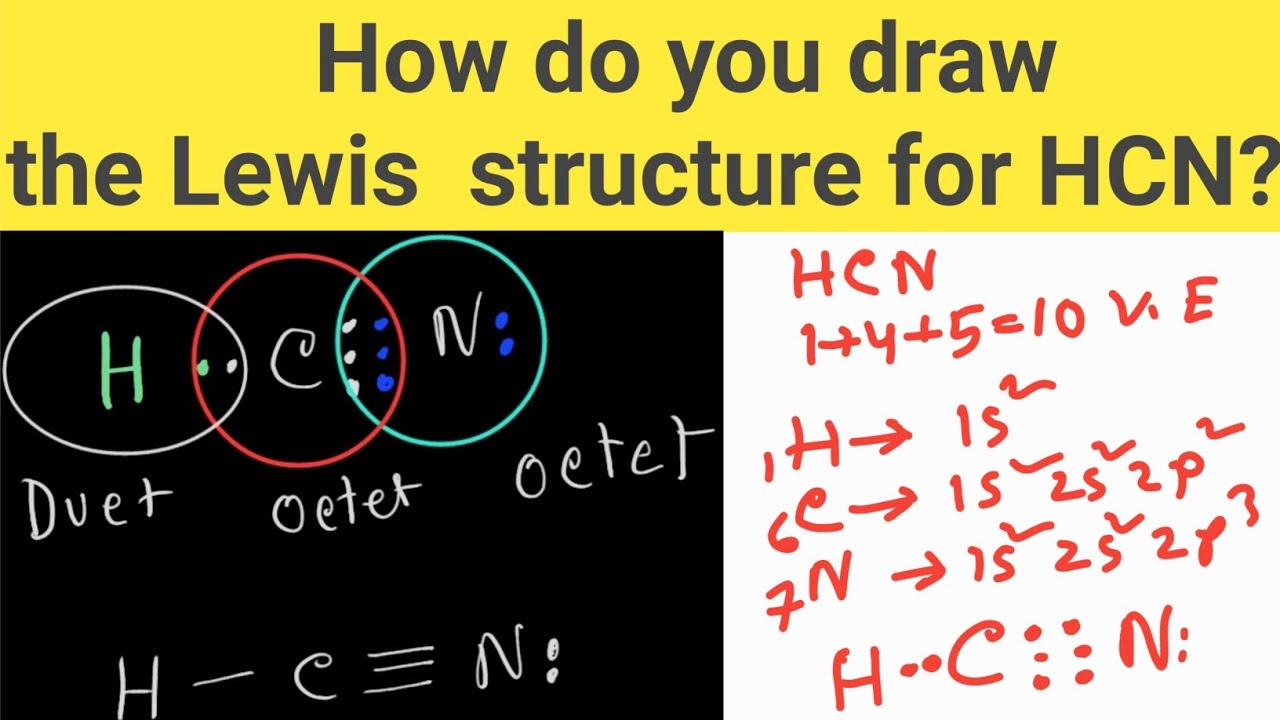
How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN Lewis Dot Structure YouTube
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

HCN Lewis Structure How to Draw the Dot Structure II lSCIENCE ll NCERT ll Rohit Sir YouTube
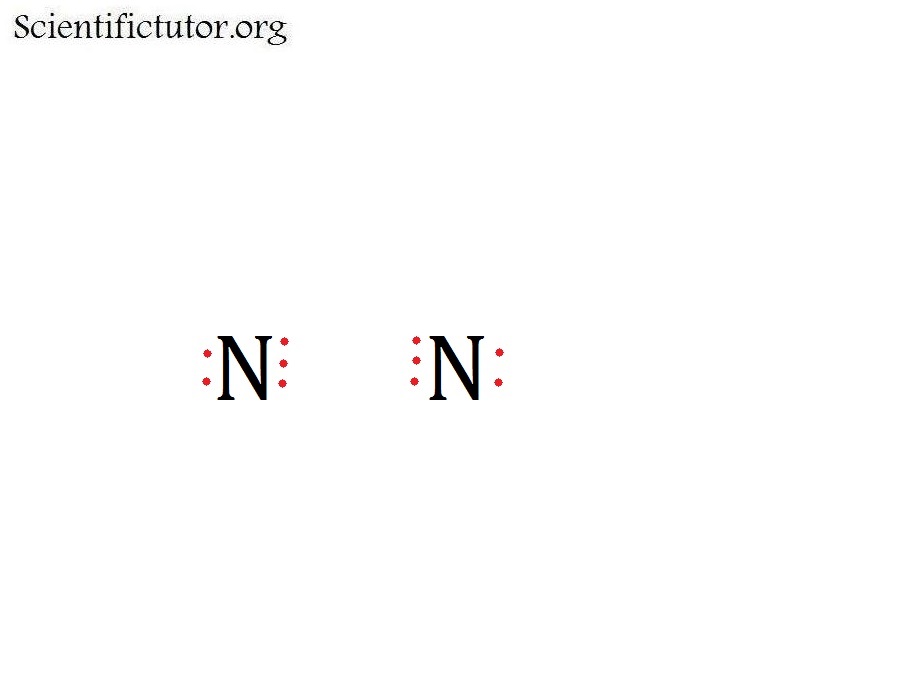
Another (easier) method to determine the Lewis structure of HCN: Alternatively a dot method can be used to draw the Lewis structure. Calculate the total valence electrons in the molecule. C : 1×4 = 4 N : 1×5 = 5 H: 1 x 1 = 1 Total = 10 valence electrons. Now, treat the atoms and electrons like puzzle pieces.